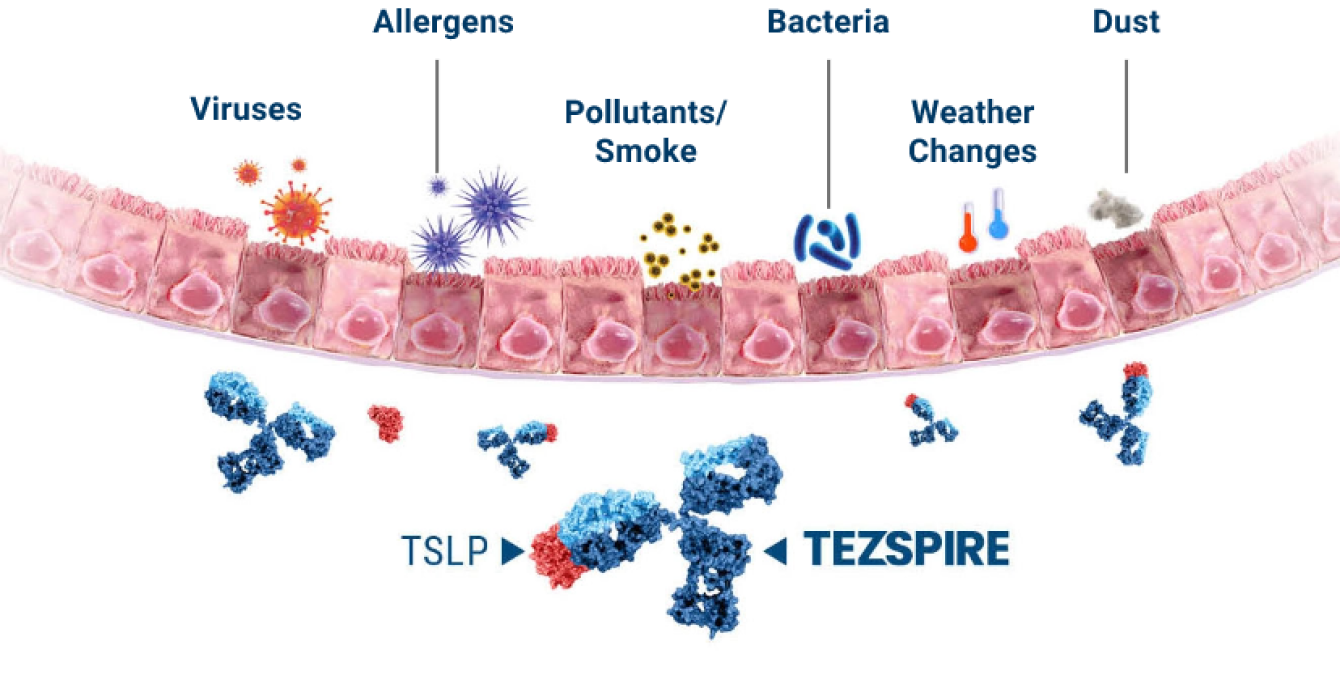
HELP PROTECT YOUR PATIENTS WITH SEVERE ASTHMA AGAINST
KEY DRIVERS OF THEIR
DISEASE
1,5,6,9,10
Results are descriptive only
Tezspire is the first and only biologic to target TSLP at the top of the inflammatory cascade 1,2,5
Impact on two hallmarks of asthma 1,5-9
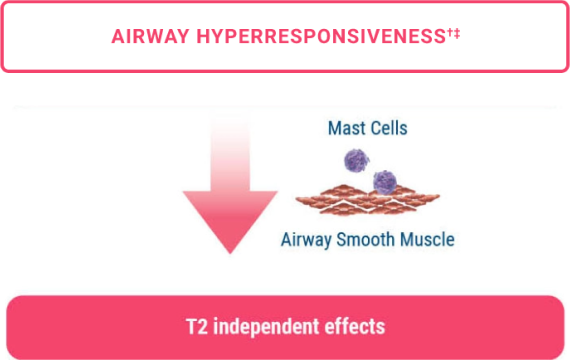
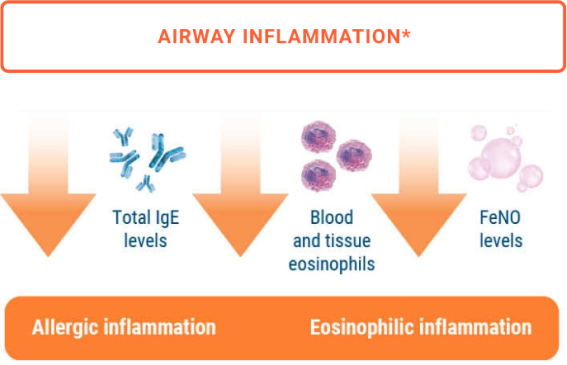
The mechanism of action of TEZSPIRE in asthma has not been definitively established.
Clinical Studies
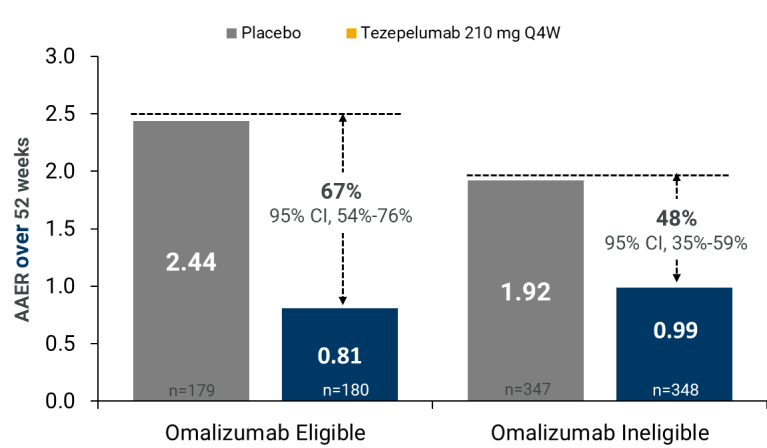
Tezspire Effect According to Eligibility for Omalizumab (EU definition)11,12
- Treatment with high-dose ICS
- Positive fluorescence enzyme immunoassaytest for classic perennial aeroallergens
- Baseline total serum IgE level of ≥30 to ≤1500 IU/mL
- Baseline body weight ≥20 to ≤150 kg
- IgE and body weight combination withinthe recommended range in the omalizumab EU label12
- AAER over 52 weeks
- Change from baseline to week 52 in:
- Pre-bronchodilator FEV1
- ACQ-6 Score
- AQLQ (S)+12 Score
- SGRQ Score
All biologics with data available reduced the AAER in patients with perennial allergy with no BEC restriction13


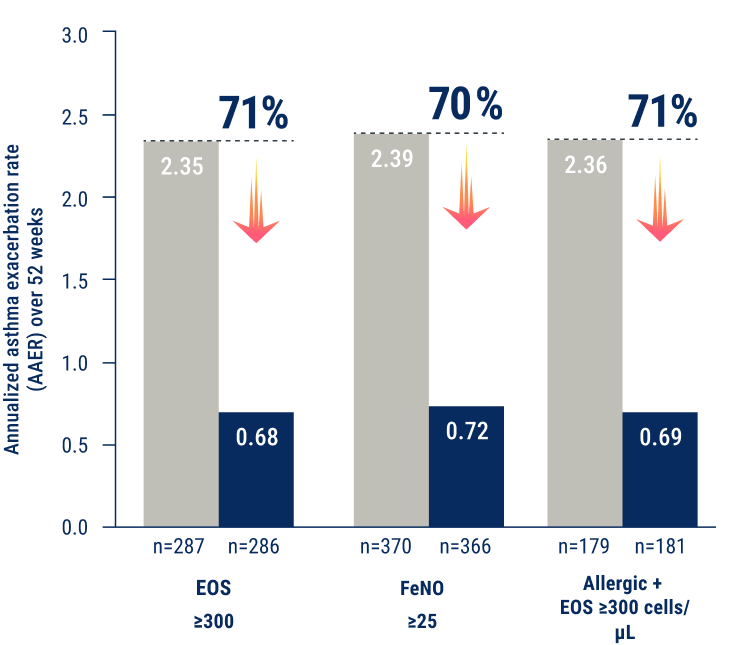
exacerbations Reductions across phenotypic profiles and biomarker levels1
Unprecedented reductions in exacerbations
TEZSPIRE consistently demonstrated statistically significant reductions in exacerbations in two 52-week pivotal trials (PATHWAY, 71%; NAVIGATOR, 56%; P<0.001).1-3*
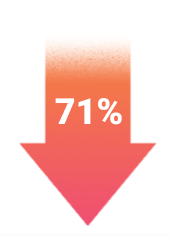
Up to 71% exacerbation reduction
in a broad, all-comer patient population1,2
P<0.001
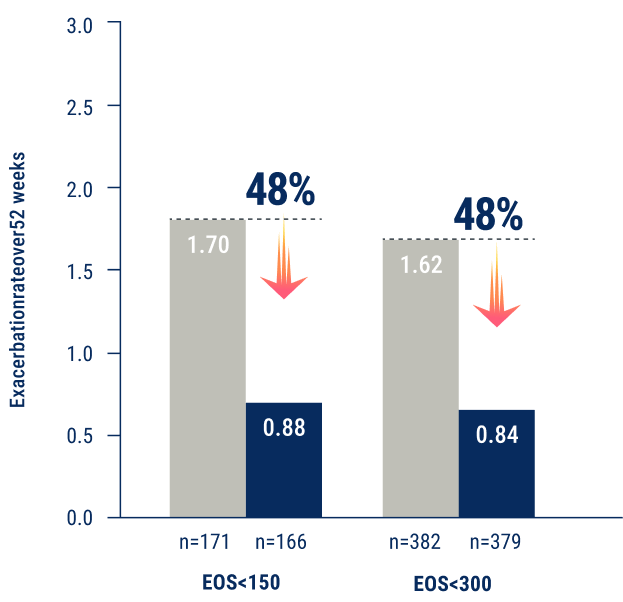
And, TEZSPIRE is the only biologic with consistent and statistically significant reduction in exacerbations in patients with eosinophils 0 to <300 cells/μL3†
Reduction in exacerbations requiring ED visits,* urgent care, or hospitalizations1,2,5
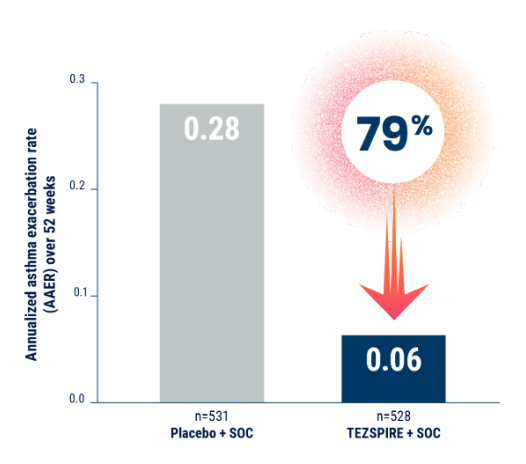
INCLUDING AN 85% REDUCTION
TEZSPIRE + SOC AAER (n=528): 0.03 Placebo + SOC AAER (n=531): 0.19 RR: 0.15 (95% CI: 0.07, 0.22)
Results are descriptive only.
Results from NAVIGATOR study.
Safety
tezspire Had an adverse event profile similar to placebo1, 10
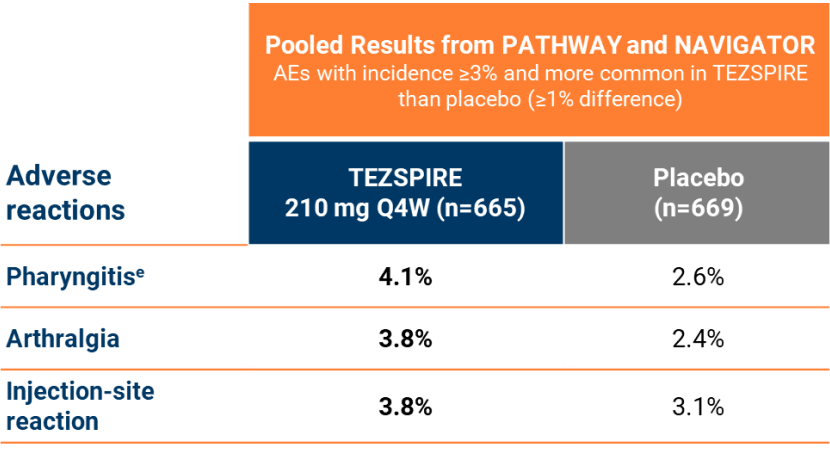
The safety profile of TEZSPIRE versus placebo was also demonstrated by:5,7
No increase risk of infection
No increase in blood eosinophils
No increase in anti-drug antibodies
The safety profile for TEZSPIRE was consistent across five randomised, placebo-controlled trials (N=1875)1,5,9,17,18
- In DESTINATION, a long-term,placebo-controlled, safety study, TEZSPIRE was well tolerated for up to 2 years, with lower incidences of overall AEs and SAEs compared with placebo and consistent with PATHWAY, NAVIGATOR and CASCADE trials5,7,9
*In a pooled analysis of PATHWAY and NAVIGATOR, AAER was 0.84 for TEZSPIRE (n=379) vs 1.62 for placebo (n=382)
in patients with baseline blood eosinophils <300 cells/μL. All patients received SOC (MD or HD ICS + additional controller).22,24,25
†48% exacerbation reduction vs placebo was consistent across both the bEOS <150 cells/µL and bEOS <300 cells/µL subgroups.
Mechanism of action
In this mechanistic study (CASCADE), airway hyperresponsiveness (AHR) to mannitol was an exploratory outcome.9
The clinical significance of this outcome and its impact on asthma have not been established.
*In the NAVIGATOR trial,5,6 patients treated with TEZSPIRE 210 mg every 4 weeks (Q4W) + SOC had least-squares (LS) mean reduction from baseline compared to placebo + SOC at week 52 in blood eosinophil counts (n=458 and n=451; -170 vs -40 cells/µL, respectively), fractional exhaled nitric oxide (FeNO) level (n=440 and n=426; -17.3 vs -3.5 ppb, respectively), and serum total IgE (n=482 and n=471; -164.4 vs +43.6 IU/mL, respectively). Results are descriptive only. In the CASCADE trial,9 the primary endpoint was the change from baseline to the end of treatment in the number of airway submucosal cells per mm2 in bronchoscopic biopsy samples. The reduction in airway submucosal eosinophils was 89% in patients treated with TEZSPIRE 210 mg Q4W + SOC (n=48) and 25% in patients treated with placebo + SOC (n=51); end of treatment to baseline geometric LS mean ratio was 0.11 vs 0.75, respectively.
‡ In CASCADE,9 AHR to mannitol was reduced in patients treated with TEZSPIRE 210 mg Q4W + SOC and placebo + SOC. There was a numerically greater reduction in AHR to mannitol in patients who received TEZSPIRE compared with those who received placebo, both in terms of absolute PD15 and in doubling doses. The clinical significance of this outcome and its impact on asthma have not been established.
CI=confidence interval; FEV1=forced expiratory volume in 1 second; IgE=immunoglobulin E; ppb=parts per billion; SOC=standard of care.
Efficacy
All biologics with data available reducedthe AAER in patients with perennial allergy with no BEC restriction
aRegardless of serum total IgE level; bRequired serum total IgE ≥ 30 IU/mL; cRequired serum total IgE 30–700 IU/mL
AAER, annualized asthma exacerbation rate; BEC, blood eosinophil count;CI, confidence interval; IgE, immunoglobulin E; Q2W, every 2 weeks; Q4W, every4 weeks; SC, subcutaneously
Tezspire Effect According to Eligibilityfor Omalizumab (EU definition)
Rate ratios are given with their 95% CIs. Percentage reductions relative toplacebo were calculated from rate ratios.aEligibilitycriteria were similar tothose reported in the EU label for omalizumab.2 AAER= annualized asthma exacerbation rate; ACQ-6 = Asthma Control Questionnaire-6;AQLQ(S)+12 = Standardized Asthma Quality of Life Questionnaire for 12 Years andOlder; ICS = inhaled corticosteroids; IgE = immunoglobulin E, Q4W = every 4weeks; SGRQ = St. George’s Respiratory Questionnaire.
Exacerbations Reductions across phenotypic profiles and biomarker levels
Post hoc analysis of pool ed PATHWAY and NAVIGATOR phenotype and biomarker subgroups.
*Allergic status as defined by a serum IgE result specific to any perennial aeroallergen in the FEIA panel.
EOS=eosinophils; FEIA=fluorescence enzyme immunoassay; FeNO=fractional exhaled nitric oxide; IgE=immunoglobulin E; ppb=parts per billion; SOC=standard of care.
Unprecedented reductions in exacerbations – up to 71% exacerbation reduction
*PATHWAY AAER: TEZSPIRE + SOC 0.20 (n=137) vs placebo + SOC 0.72 (n=138); RR: 0.29 (95% CI: 0.16-0.51); NAVIGATOR AAER: TEZSPIRE + SOC 0.93 (n=528) vs placebo + SOC 2.10 (n=531); RR: 0.44 (95% CI: 0.37-0.53).1
†Prespecified and multiplicity-protected. Results from NAVIGATOR. TEZSPIRE + SOC AAER 1.02 (n=309) vs placebo + SOC 1.73 (n=309); RR: 0.59 (95% CI, 0.46 to 0.75; P<0.001).
AAER=annualized asthma exacerbation rate; CI=confidence interval; RR=rate ratio; SOC=standard of care.
Reduction in exacerbations requiring ED visits, urgent care, or hospitalizations
*An emergency room visit was defined as evaluation and treatment for <24 hours in an ER or urgent care center that required systemic corticosteroids.6
ED=emergency department; RR=rate ratio; SOC=standard of care.
Safety PATHWAYincluded 3 tezepelumab doses; only data from the 210 mgdose are presented aPhase IIbtrial; bPhase IIItrial; cPost-hocanalysis; dNote:Clinically relevant adverse events (rash, injection-site reaction) are alsopresented; ePharyngitis(including pharyngitis, pharyngitis bacteria, pharyngitis streptococcal andviral pharyngitis);5 fRash(including rash, rash pruritic, rash erythematous, rash macular, rash papular);4,5 gPATHWAY(N=550); NAVIGATOR (N=1059); SOURCE (N=150); CASCADE (N=116); DESTINATION(N=951; 827 from NAVIGATOR and 124 from SOURCE) AE = Adverse Event; Q4W = Every 4 Weeks; SAE = Serious Adverse Event
- Tezspire approved PI by Israeli MoH.
- Gauvreau GM et al. TSLP: its role and potential as a therapeutic target in asthma. Expert Opin Ther Targets.
2020;24(8):777-792. - Lambrecht BN et al. The cytokines of asthma. Immunity. 2019;50(4):975-991.
- Lambrecht BN et al. The immunology of asthma. Nat Immunol. 2015;16(1):45-56.
- Menzies-Gow A et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;384(19):1800-1809
- Menzies-Gow A, Corren J, Bourdin A, et al. Appendix to: Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;384(19):1800-1809.
- Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377(10):936-946.
- Corren J, Parnes JR, Wang L, et al. Appendix to: Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377(suppl):1-54.
- Diver S, Khalfaoui L, Emson C, et al; CASCADE study investigators. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2021;9(11):1299-1312e7.
- Corren J, Menzies-Gow A et al; Efficacy of Tezepelumab in Severe, Uncontrolled Asthma: Pooled Analysis of the PATHWAY and NAVIGATOR Studies. AJRCCM Articles in Press. April 2023 as 0.1164/rccm.202210-2005OC by the American Thoracic Society.
- Corren J et al. Presented at: European Academy of Allergy & Clinical Immunology 2021 Hybrid Congress; July 10-12,2021; Madrid-Krakow
- Xolair approved PI by MoH
- Bernstein J. et al, Efficacy of Biologics in Patients with Allergic Severe Asthma, Overall and by Blood Eosinophil Count: A Literature Review . Adv Ther (Sep 2023) 40:4721–4740
- Menzies-Gow A et al. Poster presented at: ERS 2021 (Poster PA876);
- Jacobs JS et al. Poster presented at: AAAAI 2023 (Poster 52);
- Chowdhury NI et al. Int Forum Allergy Rhinol. 2017;7:1149–1155;
- Wechsler M et al. Lancet Respir Med.2022;10:650-660
- Menzies-Gow A et al. Lancet RespirMed.2023 [Epub ahead of print]
- Nucala prescribing information approved by MoH
- Cinqair prescribing information approved by MoH.
- Fasenra prescribing information approved by MoH.
- Dupixent prescribing information approved by MoH.
- Xolair prescribing information approved by MoH.
- Wechsler ME, Colice G, Griffiths JM, et al. SOURCE: a phase 3, multicentre, randomized, double-blind, p lacebo-controlled, parallel group trial to evaluate the efficacy and safety of tezepelumab in reducing oral corticosteroid use in adults with oral corticosteroid dependent asthma. Respir Res. 2020;21(1):264.
האתר מיועד לקהל רפואי מקצועי בלבד
אסטרהזניקה 2025 כל הזכויות שמורות
רח׳ עתירי ידע 1 , בניין O-TECH 2 , קומה 5 – ת.ד. 8044 כפר סבא 4464301, טל. 073-2226099
IL-5419 | Aug 2025